Table of Contents
- Introduction:
- Conjugation of oligonucleotides to antibodies: 9
- Challenges and Solutions for Process Development, Manufacturing, and Characterization of AOC:
- Unique Pathways to Perform AOCs Process Development and Manufacturing:
- Typical conjugation performed at GBI for bio-conjugates may include the following (See Table 1):
- GBI’s Antibody-Oligonucleotide Process Development and GMP Manufacturing Requirements:
- Conclusion and Future Outlook:
- References
Authors:
Muctarr Sesay, PhD, Chief Scientific Officer (GBI Biomanufacturing Inc.)
Natalia Bourguignon, PhD, Manager, MSAT USP (GBI Biomanufacturing Inc.)
Hyung-Il Lee, Ph.D, Senior Scientist Bioconjugation Development (GBI Biomanufacturing Inc.)
Introduction:
In recent years, oligonucleotide-based therapy has gained attention as an emerging class of target therapeutic agents by inhibiting specific genes involved in various diseases including cancers, viral infections, and genetic disorders. These oligonucleotides include single-stranded DNA (antisense oligonucleotide: ASO) or RNA (miRNA), and double-stranded RNA known as small interfering RNA (siRNA). ASO can activate RNA degradation through the induction of endogenous RNases or modulate gene splicing by exon skipping or inclusion. In contrast, siRNAs and miRNAs utilize the intrinsic RNA interference pathway to degrade RNAs or inhibit translation. Because of the target specificity, siRNAs have been used more as therapeutic tools. The therapeutic effect of siRNAs has been shown to be better than that of peptides or inhibitors.1 However, siRNAs need to overcome two major obstacles before they can be employed therapeutically. First, unmodified siRNAs have low stability in serum and non-specific accumulation in cells. Second, siRNAs have poor cell membrane permeability due to their high negative charge and large sizes.2 Recently, the stability of siRNAs in blood has been improved by RNA delivery vehicles such as polyethyleneimine (PEI), liposome, and N-acetylgalactosamine (GalNAc). To date, four siRNA drugs utilizing RNA delivery vehicles have been approved by the FDA. Patisiran (ONPATTRO®) was the first approved liposomal siRNA drug in 2018 for treating patients with hereditary ATTR (hATTR) amyloidosis. Givosiran (GIVLAARI®) was the second approved GalNAc‐linked siRNA drug in 2019 for the treatment of patients with acute hepatic porphyria (AHP). Two more GalNAc‐linked siRNAs approved in 2020 were Lumasiran (OXLUMO®) and Inclisiran (LEQVIO®) for the treatment of patients with primary hyperoxaluria type 1 (PH1) and hypercholesterolemia, respectively.3,4,5,6 According to the market analysis by Research Nester Pvt. Ltd., the market for siRNA is expected to exceed $67 billion by the end of 2036, with an annual growth rate of 18.5% between 2024 to 2036. 7
The delivery of all approved siRNA-based drugs and many other siRNAs studied has been mainly directed to the liver. Despite advances in improved stability and efficient delivery of siRNAs to the liver, delivery of siRNAs to other tissues and organs has been challenging and only a few siRNAs have advanced to clinical trials. RXI-109, which has completed Phase 2, is a modified siRNA to reduce hypertrophic scarring and keloid formation. In more recent years, monoclonal antibodies (mAbs) have been employed for siRNA delivery vehicles due to their specificity to antigens expressed on target cell surfaces. Monoclonal antibodies have already been used to deliver small molecules to the targeted tumor cells via the receptor-mediated internalization process as antibody-drug conjugates (ADC). Since the FDA approved the first ADC, gemtuzumab ozogamicin (MYLOTARG®), in 2000 for the treatment of patients with acute myeloid leukemia (AML), fourteen more ADCs have been commercialized to treat various cancers. The successful application of ADCs in oncology has expanded to developing antibody oligonucleotide conjugates (AOCs, Figure 1). Initially, AOCs were used as diagnostic tools but more recently as targeted therapeutic agents.8 The ability of the antibody portion of AOCs to deliver to specific tissues or cells overcomes the limit of unmodified oligonucleotides that are delivered mostly toward the liver. Also, the interaction of the endocytic antibody portion with epitopes on the surface of the target cells allows AOCs to be internalized. Furthermore, in vitro stability studies showed that the oligonucleotide portion of the AOC was more stable than the oligonucleotide alone.
Note that since AOCs are still in their infancy for therapeutic applications for modalities such as cancer, very little to no market research information is available indicating the potential for this promising technology.
Conjugation of oligonucleotides to antibodies: 9
Currently, AOCs are generated mostly by three methods: 1) electrostatic interaction-based conjugation, 2) avidin-biotin affinity-based conjugation, and 3) covalent binding-based conjugation.
Positively charged protamines linked to antibodies have been used to bind negatively charged siRNAs to form antibody siRNA conjugates via ionic interactions. This method has been applied to suppress HIV, inhibit prostate cancer cells, treat arthritis, and treat myasthenia gravis (autoimmune disease). The advantage of this method is the polycationic complex can escape from lysosomes by osmotic swelling. However, due to the reversibility of the interactions, the conjugate could be dissociated under changing pH or salt concentration.
- (2) Avidin-biotin affinity-based conjugation
AOCs have been prepared using the strong interactions between a biotin-labeled oligonucleotide and avidin-linked antibody. This method was applied to treat wide variety of cancer and podocyte-injured glomerular diseases. Because of the complexity of chemical modification of the antibody and oligonucleotide, this method has been used rarely for conjugation compared to ionic interactions and direct conjugation.
- (3) Direct chemical conjugation (See Figure 1)
The linker chemistries and conjugation methods for AOCs are analogous to the methods used for generating ADCs (antibody drug conjugates) and other bio-conjugates. The most common approach to preparing AOCs is to link oligonucleotides to either lysine residues on antibodies or cysteine residues that are reduced from the disulfide bonds using reducing agents. These conjugation methods do not require modification of antibodies but result in heterogeneity of conjugates with various oligonucleotide antibody ratios (OAR), a parameter used to describe the ratio of oligonucleotides to antibodies. Thus, the OAR and sites of oligonucleotide conjugation can impact the pharmacokinetics and therapeutic index of AOCs. To overcome heterogeneity, homogeneous AOCs have been developed using site-specific strategies. One approach to creating site-specific AOCs is the THIOMABs technology developed by Genentech. In this method, the site-specific cysteine-engineered antibodies provide a defined antibody:siRNA stoichiometry. The AOCs generated using this platform showed intratumoral delivery and gene silencing effects in a mouse model. The drawback of this method is the stability of conjugates. The thiosuccinimidyl linkage is reversible and can undergo premature cleavage upon exchange with circulation thiols in vivo. The other approach is to use click chemistry which is the Cu(I)-catalyzed 1,3-dipolar azide-alkyne cycloaddition between azides and alkynes (CuAAC). Because of the robustness and selectivity of click chemistry, this method has been used to synthesize various conjugates. The disadvantage of this method is that copper ions may denature proteins. This issue was resolved by the development of strain-promoted alkyne-azide cycloaddition (SPAAC) which is Cu-free click chemistry. In SPAAC, a dibenzocyclooctyne (DBCO) moiety of the antibody reacts covalently with an azide-modified oligonucleotide. This reaction has no adverse effects on antibodies.
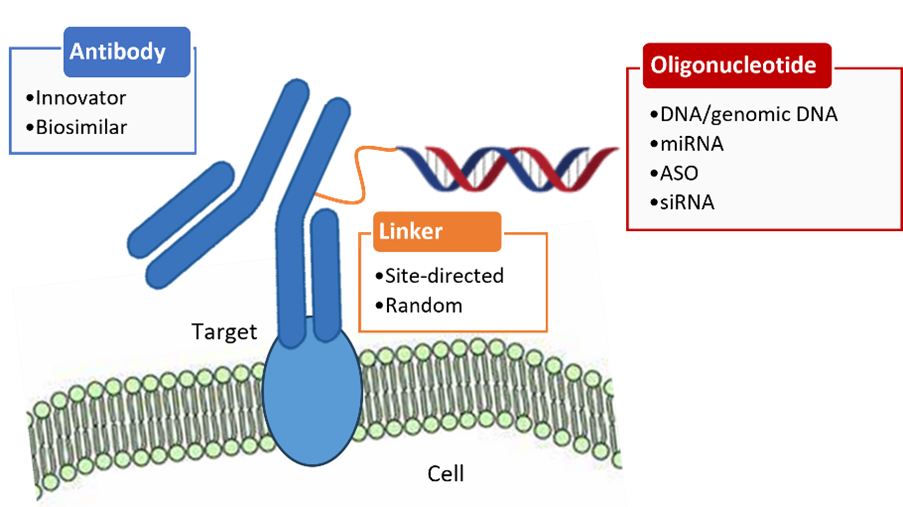
Figure 1: Scheme of antibody oligonucleotide conjugates. miRNA: microRNA, ASO: antisense,
siRNA: small interfering RNA.
Challenges and Solutions for Process Development, Manufacturing, and Characterization of AOC:
The application of AOCs as biotherapeutics is an emerging field, and due to their structural characteristics, some manufacturing steps as well as analytical methods for their characterization need to be modified or developed to address the production for clinical trials. Some considerations for the synthesis of AOCs are as follows:
- The analytical and purification methods will be developed specifically for AOC because an oligonucleotide is larger than a typical drug-linker and can have a MW greater than 10 kDa. This means that the oligonucleotide has a larger influence on the physical and chemical properties of the conjugated antibody (150 kDa) than small molecules. 9
- The analysis and characterization need to be adapted to the AOC compared with the antibody and modified antibody. The oligonucleotides are negatively charged with phosphate backbones (with over 20 negative charges), they are water soluble and will dominate the overall charge profile of the AOC.
- OARs determination. Chromatographic methods, which rely on the hydrophobicity of the drug-linker, are not transferable to OAR, and it is usually accomplished by mass-spectrometry, fluorescence, or PCR amplification.
- Safety and facilities are important. Solvents used in oligo-based drug manufacturing can be hazardous. Manufacturers must provide safe transport to the facility and guarantee safe on-site storage. Cold storage needs to be available to keep products and intermediate compounds.
- Explore the frozen storage temperature of the AOC if the product is not stable refrigerated (4ºC) rather than lyophilized powder during the early stages of clinical trials.
- For stable final formulation buffer , the utilization of appropriate excipients and/or additives may be needed to preserve the AOC stability and functionality.
Unique Pathways to Perform AOCs Process Development and Manufacturing:
AOCs are a novel class of synthetic chimeric biomolecules that have been continually gaining traction in modern biotechnological applications. This is primarily due to the unique combination of the properties of their two main constituents, exceptional targeting abilities, and biodistribution profiles of the antibody (biosimilar and/or innovative) in addition to an extensive scope of the oligonucleotide functional and structural roles in terms of potency and precision therapies to treat previously untreatable diseases. In this article, as a flexible manufacturing CDMO, we are presenting this yet emerging class of chimeric biomolecules AOCs overview of the practical considerations regarding process development, GMP manufacturing, characterization, and IND enabling activities for early Phase clinical trials. A general overview of the field of oligonucleotides with other peptides, proteins, and small molecules is provided in recent review articles.9,10 Furthermore, GBI has end-to-end capabilities and experience in performing process development and manufacturing of numerous monoclonal antibodies and the corresponding antibody-ligand conjugates.11,12,13,14,15,16
Antibody-oligonucleotide conjugates have more challenges and more conjugation methods than typical conjugation of small molecules in Antibody-drug conjugates (ADC). As described earlier, the methods used to prepare AOCs include electrostatic interactions, the affinity between biotin and avidin, and direct conjugation to the antibody. The covalent methods for the synthesis of AOCs enable stable linkages and afford more defined structures making them appropriate for therapeutic applications. On the covalent approaches the oligonucleotide (ON) moiety can be chemically coupled with free primary amine or be transformed into a more reactive thiol. The appropriate bifunctional chemical linkers are usually chosen by the reactive group present in the antibody structure (native vs engineered) and the accessibility of ON derivatives.2,9
Typical conjugation performed at GBI for bio-conjugates may include the following (See Table 1):
- (1) Oligonucleotide modification: This requires the covalent coupling of the oligonucleotide with an appropriate heterobifunctional crosslinking agent to afford the oligonucleotide-linker. The linker design requires to be stable in circulation and upon antibody-mediated internalization, the payload is efficiently released. Reactive groups such as maleimide, thiol, NHS-Ester and other functional groups are made available on the unconjugated end of the linker for conjugating onto the antibody
- (2) Antibody conjugation onto the activated linker-oligonucleotide conjugate: Several approaches can be employed to conjugate an antibody onto the activated linker-oligonucleotide payload.10,13,14,15
- (a) GBI’s novel site directed conjugation of the antibody via the disulfide bridge at the hinge region and away from the hypervariable antigen binding region. This involves the reduction of the one of the disulfide bridges of the antibody via a non-thiol reducing agent such as TCEP. The resulting in the formation of two thiol (-SH) reactive groups. This is followed by coupling with the Maleimide-Linker-Oligonucleotide conjugate to afford the antibody-Linker-Oligonucleotide product via a thio-ether linkage. An OAR of 2: 1 of the Oligonucleotide: Antibody is achieved. 15,16
- (b) Randon conjugation of the amine reactive antibody with the activated (via NHS-Ester) linker-oligonucleotide conjugate.
- (c) Direct conjugation (no linker) of the antibody and the ON through carbodiimide (EDC)/NHS-Ester chemistry.
GBI’s Antibody-Oligonucleotide Process Development and GMP Manufacturing Requirements:
A project for GMP manufacturing of AOC at GBI starts with the assessment of the technology and clinical manufacturing. This assessment involves the tech transfer of data by the client. The data to be analyzed will include a) chemical (small molecule payload) and protein (antibody) conjugation processes and characterization. Safety, equipment and facility considerations (with organic solvents); b) Target parameters for process optimization such as mole ratio of antibody:oligonucleotide; mixing studies, conjugation temperature and time, process economics, compliance, scalability among others; and c) Investigational New Drug (IND) enabling activity requirements for CMC submission to the regulatory authorities such as product stability (via ICH guidelines), appropriate product release assays (safety, identity, potency, purity, overall quality) and creation of a reference standard.
A typical end-to-end project usually involves antibody production, beginning from a research cell bank (RCB). This run comprises of process development (PD), master cell bank (MCB) creation, and GMP manufacturing of the antibody. This process generally takes about 8 months using GBI’s antibody manufacturing platform and has been well described in our previous articles (Step 1 in Figure 2). 13,17 An alternative approach is for the client to provide a commercially available biosimilar for the antibody.
The project continues with the PD and GMP manufacturing of the Oligonucleotide-linker, which is a conjugate intermediate, taking approximately 2 months (Step 2, Figure 2).
The last stage is the PD and GMP run for the AOC conjugation where several options and approaches are available at GBI and as described in the previous section. The time for AOC conjugation is about 3 months (Step 3, Figure 2). The resulting AOC drug substance (after formulation) is filled aseptically to afford the drug product.
The total end-to-end timeline proposed by GBI is about 10-12 months considering that some of the activities may occur in parallel. GBI as a flexible CDMO can also partially execute the project, depending on the client’s needs.
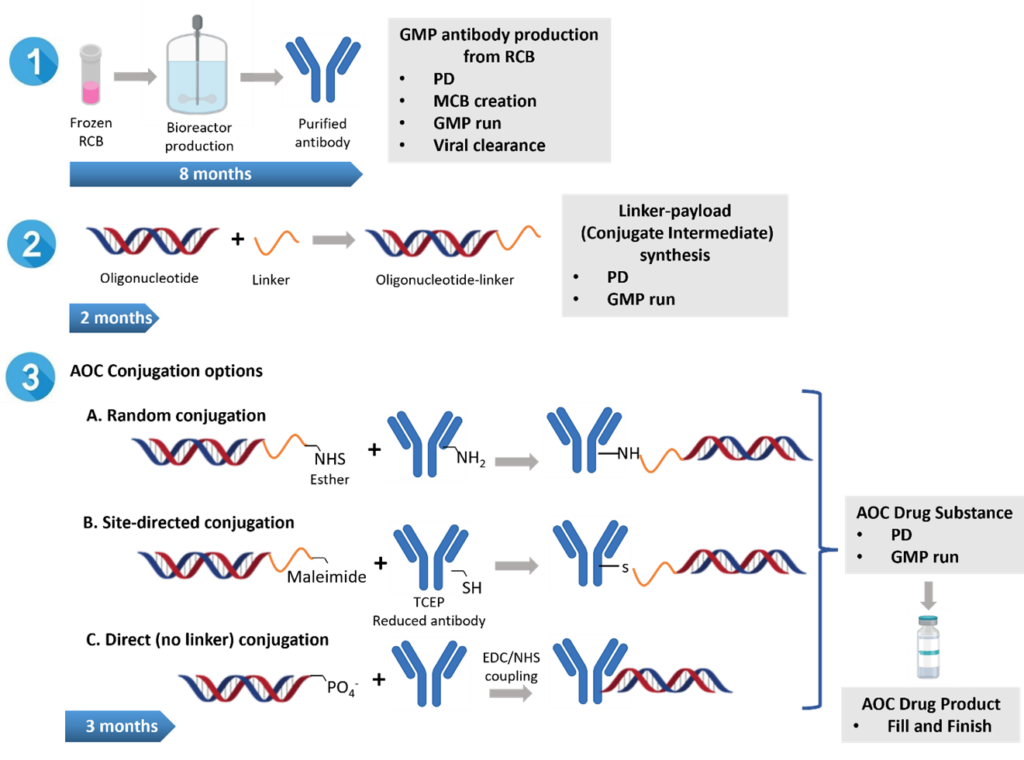
Figure 2: Manufacturing of antibody oligonucleotide conjugate (AOC) at GBI. Streps 1, 2 and 3 for process development, manufacturing and IND enabling activities may overlap while the purified antibody, oligonucleotide and linker are available. RCB: Research Cell Bank, PD: process development, MCB: Master Cell Bank, GMP: Good Manufacturing Practices, TCEP: Tris(2-carboxyethy1)phosphine, EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, NHS: N-hydroxysuccinimide.
GBI has been developing and manufacturing a wide range of antibody-drug conjugates for more than two decades. Table 1 depicts recent antibody conjugates with many types of payloads including oligonucleotide and genomic DNA.
Table 1: Recent Selected Antibodies Conjugates Manufactured by GBI
| IgG Monoclonal Antibody : DOTA Chelator (In111 Isotope) | Process Development and GMP |
| IgG Monoclonal Antibody: CHX-A” Chelator (In111 Isotope) | Process Development and GMP |
| IgG Monoclonal Antibody : CHX-A” Chelator (Y90 Isotope) | GMP Manufacturing |
| IgG Monoclonal Antibody : Conjugation of I131 Isotope | GMP Manufacturing |
| Recombinant Fusion Protein : DOTA Chelator (Cu64 Isotope) | GMP Manufacturing |
| IgG Monoclonal Antibody: Fluorescent Dye (Alexa Fluor) | Process Development |
| Recombinant Protein : Small Molecule Ligand | Process Development |
| IgG Monoclonal Antibody : Fluorescent Dye (IR700) | GMP Manufacturing |
| IgG Monoclonal Antibody Fragment: Peptide | Process Development |
| Recombinant Fusion Protein : DOTA Chelator (Cu64 Isotope) | GMP Manufacturing |
| IgG Monoclonal Antibody: Fluorescent Dye (Alexa Fluor) | Process Development |
| Recombinant Protein : Small Molecule Ligand | Process Development |
| IgG Monoclonal Antibody : Fluorescent Dye (IR700) | GMP Manufacturing |
| IgG Monoclonal Antibody Fragment : Peptide | Process Development and GMP |
| Glycosaminoglycan: Peptide | Process Development |
| IgG Monoclonal Antibody : Fluorescent Dye (IR800) | Process Development |
| Antibody-Oligonucleotide | Process Development |
| Antibody-Genomic DNA | GMP manufacturing |
| Opioid Vaccine Conjugates | GMP manufacturing |
Conclusion and Future Outlook:
The field of AOCs has recently received increasing attention as an emerging class of functionalized antibodies that have already been used in a wide range of applications. Combining the specific binding abilities of antibodies with the vast structural and functional properties of oligonucleotides, these conjugates have found a wide variety of applications as imaging, detection, and a versatile class of chimeric biomolecules for therapeutic agents. It is envisioned that the landscape of AOCs will evolve rapidly over the next decade or so as the field of ASO, siRNA, miRNA and other oligonucleotides advances. This evolution includes addressing major challenges associated with analytical characterization, target and disease selection, drug trafficking, in vivo stability knowledge and mechanistic understanding of existing and new AOC constructs. With over 20 years of experience and knowledge of bio-conjugates (including nucleic acid conjugates), GBI as an end-to-end CDMO is uniquely qualified to partner with prospective clients and researchers in the process development, GMP manufacturing, characterization, and pertinent IND and BLA enabling activities of the “naked” mAb (from the RCB), linker-oligonucleotide conjugates, antibody-linker-oligonucleotide conjugates drug substances and aseptic fill/finish to afford the vialed drug product.
References
1.Ibtehaj N, Huda R. (2017). “High-dose BAFF receptor specific mAb-siRNA conjugate generates Fas-expressing B cells in lymph nodes and high-affinity serum autoantibody in a myasthenia mouse model”. Clinical Immunology 176, 122-130
2. Baumer N, Berdel WE, Baumer S. (2017). “Immunoprotein-mediated siRNA delivery”. Molsiv Pharm 14(5), 1339–51.
3. Lee SK et al. (2012). “Cell-specific siRNA delivery by peptides and antibodies”. Methods Enzymology 502, 91–122.
4. Chen X et al. (2018). “RNA interference-based therapy and its delivery systems”. Cancer Metastasis Rev 37(1), 107–24.
5. Hoy SM. (2018). “Patisiran: First global approval “. Drugs. 78(15), 1625–31.
6. Scott LJ. (2020). “Givosiran: First approval”. Drugs. 80(3):335–9.
7. Nagar, S. (2024) “ A Look At siRNA & Market Trends ”. Advancing RNA. 3 Jan
8. Mullard, A. (2022). “Antibody-oligonucleotide conjugates enter the clinic”. Nat. Rev. Drug Discov, 21(1), pp.6-8.
9. Dugal-Tessier J. Thirumalairajan A., Jain N. (2021). “Antibody-Oligonucleotide Conjugates: A twist to Antibody-Drug Conjugates”. J. Clin Med, 10(4), 838
10. Dovgan, I., Koniev, O., Kolodych, S., & Wagner, A. (2019). “Antibody–oligonucleotide conjugates as therapeutic, imaging, and detection agents”. Bioconjugate Chemistry, 30(10), 2483-2501.
11. Sesay, M. (2022). “Vaccines Present a Novel Strategy for Opioid Overdoes Prevention” Pharma’s Almanac. 31 Oct..
12. Sesay, M. “Theranostic Manufacturing Solutions.” Pharma’s Almanac. 29 Aug. 2022
13. Sesay, M and Majdoch, A. “Leveraging Experience to Reduce Timelines for IND-Enabling Activities.” Pharma’s Almanac. 19 Sep. 2022
14. Pincus, S. et.al. (2002) “Evaluation of Antigen-Based Heteropolymer for Treatment of Systemic Lupus Erythematosus in a Nonhuman Primate Model” Clinical Immunology, 105 (2), 141-154
15. Tati, S et al. (2017) “Humanization of JAA-F11, a Highly Specific Anti-Thomsen-Friedenreich Pancarcinoma Antibody and In Vitro Efficacy Analysis.” Neoplasia, 19(9), 716-733
16. Revskaya, E et.al. (2017) “A Radiolabeled Fully Human Antibody to Human Aspartyl (Asparaginyl) beta-Hydrolase is a Promising Agent for Imaging and Therapy of Metastatic Breast Cancer”. Cancer Biotherapeutics and Radiopharmaceuticals, 32 (2), 57-65
17. Sesay, M and Bourguignon, N. (2024) “Expediting Radioimmune Conjugate Pharmaceuticals for First-In-Human Phase 0 Proof-of-Concept Human Studies from Stable Pool”. Pharma’s Almanac. 15 Mar
