
Experience.
Opportunity.
Biosimilars & Biobetters
Many branded biologics with annual sales of over $50 billion are facing expired or soon-to-be expired patent protections. This represents a significant opportunity for developing biosimilars.
But biosimilar development has unique challenges of complexity and cost; unlike innovative biomolecules, biosimilars need to be fully characterized with pharmacology and toxicology studies followed by clinical trials.
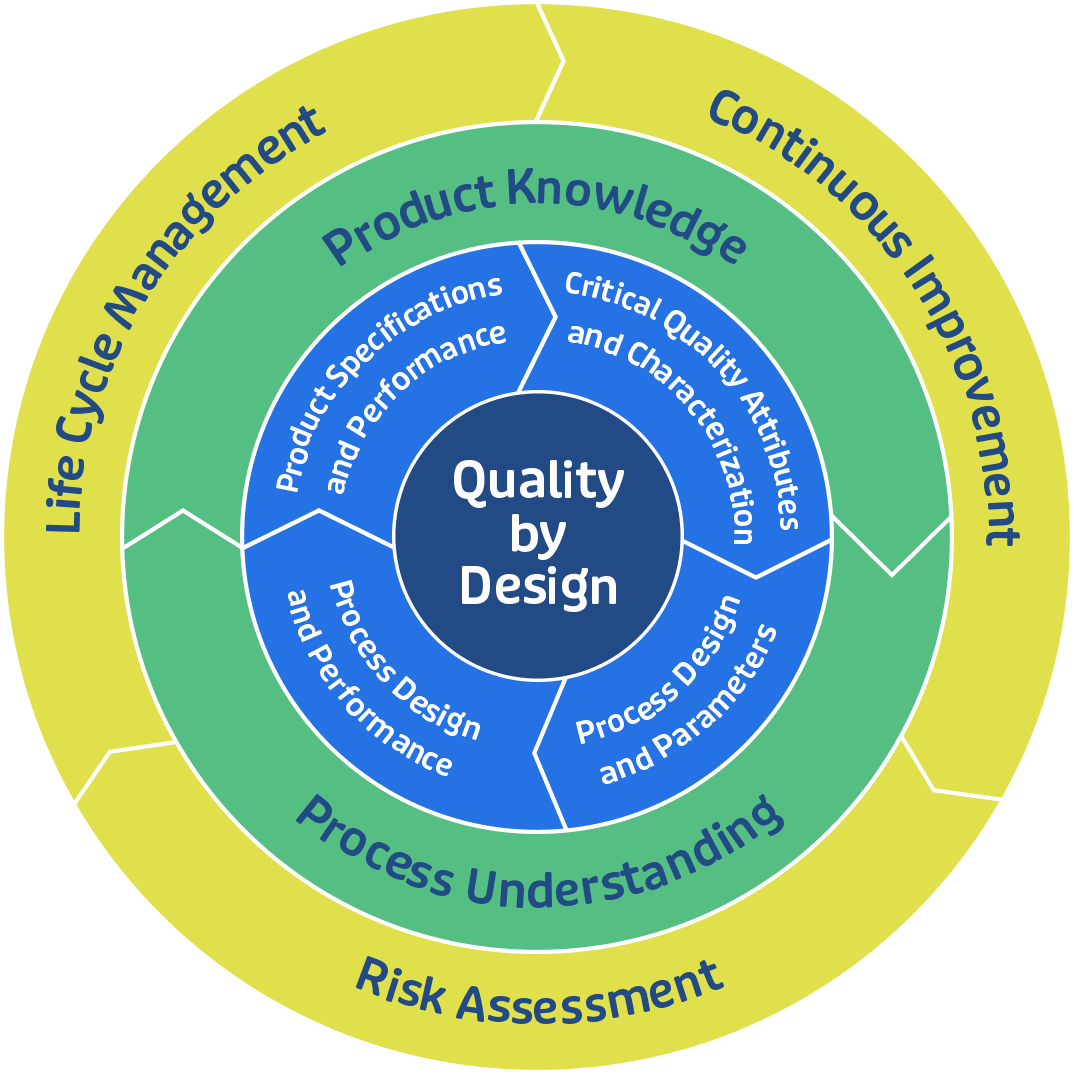
Developing and commercializing a biosimilar can take several years. There is a large burden of proof to establish that the biosimilar is highly similar to the innovative protein to which it is being compared. Since the passing of the 2009 Biologics Price Competition and Innovation Act (BPCIA), the industry has had a legislative pathway to the approval of biosimilars. In response the FDA has implemented the concept of Quality by Design (QbD), a systematic and science-driven approach demanding a “totality of evidence” to establish similarity using process parameters and controls and product specifications and quality attributes.
That’s why GBI has built expertise in designing your approach for characterization and comparability studies of the innovator drug product and your biosimilar drug candidate.
We work to understand the reference product very thoroughly so that you have the correct targets for identity and purity profiles and can use our wide variety of analytical assays, full characterization, and stability studies to establish that your drug candidate conforms to the known quality and established reference standards.
Like every project at GBI, biosimilar and biobetter development integrates risk analysis to optimize product quality, assessing critical quality attributes like aggregate formation, sub-visible or visible particles, the presence of certain sugars, host cell proteins, residual DNA, and others. Additional risk assessment may be performed on the product’s formulation composition, including pH, antibody concentration, detergent concentration, buffer salt concentration, primary container, raw material impurities, and others.
We will work with you to develop a strategy to identify regulatory pathways and gain regulatory approval of your biosimilar in accordance with the FDA, EMA, WHO, DCGI, and others.

Experience.
Specificity.
Complex Biologics
With a growing focus in biopharmaceuticals on the development of complex biologics that allow the targeted delivery of products with multiple functionalities, bi- and multi-specific antibodies, antibody fragments, and recombinant proteins have become recognized as prime examples of new drug substances with the potential to offer an increased safety profile and higher potency.
Because the closeness of the binding sites (and their increased numbers) lends to the formation of new complexes and the triggering of new cellular contacts, multi-specifics have the potential to be more precisely targeting and potent than conventional mAbs or combination therapies. Multi-specifics can also encourage the activation of additional immune responses by T cells and natural killer cells, leading to increased targeted cytotoxic effects against tumors.
Aggregate Removal Development and GMP Manufacturing for a Conjugated Theranostic Product
Download
Experience.
Innovation.
Exosomes
Exosomes are novel biotherapeutics, but their manufacture is similar to that of conventional biologics in terms of cell culture and bioreactor (upstream) development, as well as purification (downstream) development. The challenge of exosome production lies not only in the manufacture of these biologics, but also in their characterization.
GBI has extensive experience with producing, purifying, and characterizing exosomes, which are found in most bodily fluids and have been shown to play key roles in processes like coagulation, intercellular signaling, immune responses, and cellular waste management. Broad exosome applications include the delivery of biologics payloads to cells such as DNA, mRNA, peptides, and proteins; the therapeutic treatment of COVID-19, cancers, and rare diseases; and the formulation of cosmeceuticals.
Most developers of these innovative technologies are in pre-clinical and early clinical stages as they work to demonstrate the efficacy and potential of exosome-based treatments and products.





