

Experience.
Agility.
Bioconjugation Services by a Proven US-Based CDMO
As a top-tier US-based CDMO, GBI offers comprehensive bioconjugation services to support the full life cycle of antibody conjugates and complex biologics. Our proprietary Single-Source Solution™ integrates proof of concept, development, optimization, and cGMP manufacturing of bio-conjugates under one roof—streamlining timelines, and process economics and complaince for clinical and commercial programs therapeutics and diagnostic applications.
With deep expertise in conjugation chemistries and process scale-up strtegies, we’re a go-to partner for biotech and pharma innovators seeking CDMO companies in the US with the specialized capabilities to bring conjugated biologics to market faster.
Contact UsFull-Service Bioconjugation for Bioconjugates
From early-stage drug product development through to commercial readiness, GBI provides end-to-end support for a wide range of bioconjugate modalities:
- Radioimmune conjugates (Theranostics and Imaging)
- Photoimmune conjugates
- Antibody oligonucleotide conjugates
- Vaccine conjugates
- Controlled substances conjugates (opioid addiction)
- Pegylation conjugates
- Other ligand (payload) conjugates
Our cGMP manufacturer capabilities ensure your program meets all regulatory and quality requirements for clinical trial material and commercial supply.
20+ Years of Bioconjugation Expertise
Since 2002, GBI has completed over 50 bioconjugation projects—from proof of concept through process development, and cGMP scale-up and manufacturing for clinical and commercial use.
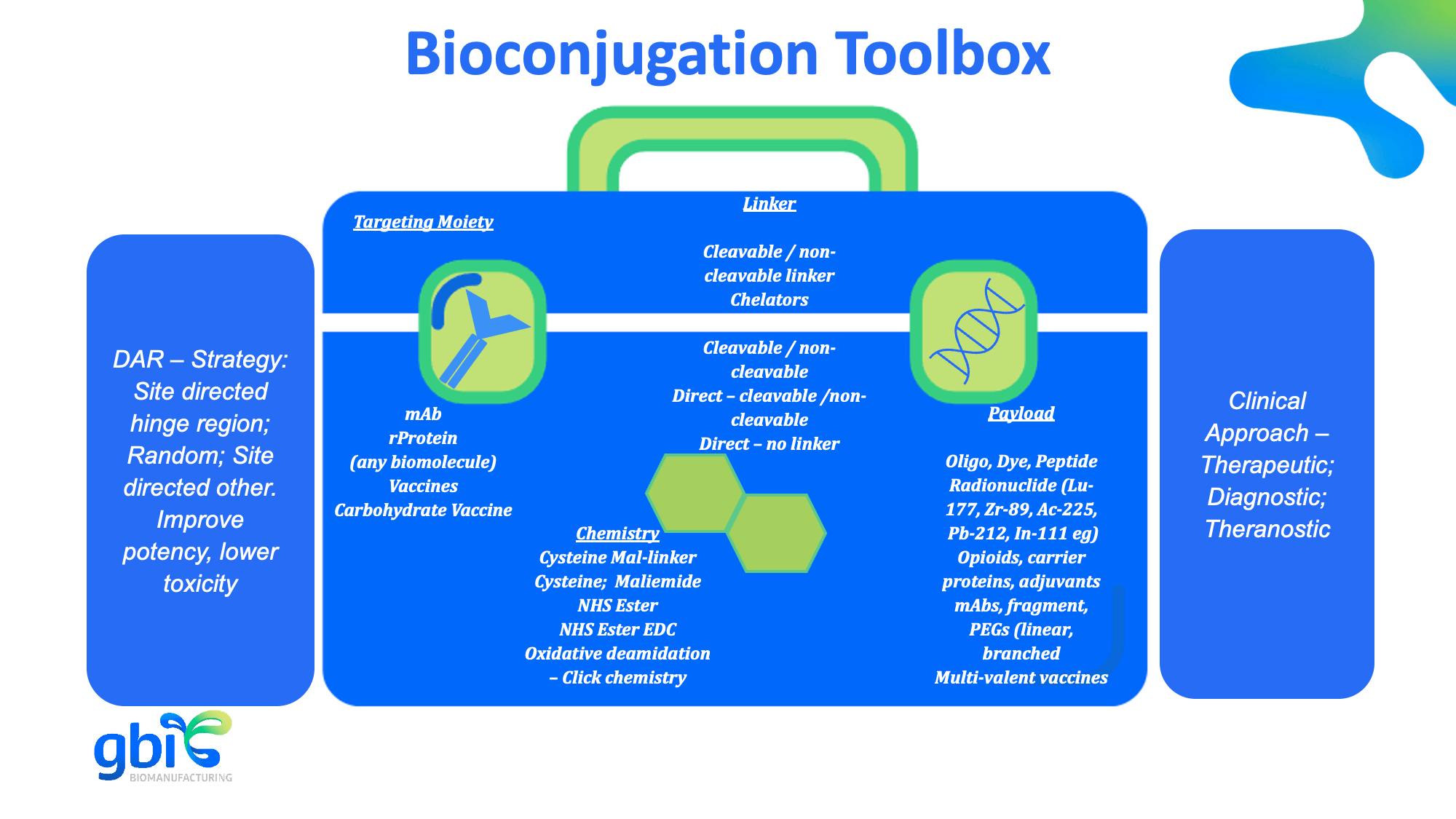
Our bioconjugation portfolio includes:
- ADC development with cleavable and non-cleavable linkers
- Conjugation to carrier proteins (e.g., KLH, CRM197) for immunogen production
- Opioid conjugate vaccines
- Multi specific antibody, antibody fragment and recombinant proteins conjugation
- Metal chelate labeling for radiopharmaceuticals (including IgGs and IgMs)
- Biotinylation, peptide and fluorescent dye labeling
- Conjugation of genomic DNA and oligonucleotide to antibodies
- Adsorption of adjuvants like aluminum hydroxide (and other client specific adjuvants) onto antibodies
Our development team understands the critical nuances of bioconjugate optimization and scale-up —such as active site masking, isotope uptake, and conjugation efficiency—making us a trusted CDMO US companies rely on for complex conjugation workflows.
Schedule I and II Controlled Substance Manufacturing
As a DEA-licensed Schedule I and II manufacturer, GBI is uniquely equipped to develop and manufacture conjugate vaccines containing controlled substances. This includes projects like opioid addiction vaccines, for which we’ve successfully delivered cGMP-compliant materials for preclinical and clinical studies.

We have selected GBI based on the years of experience they have in bioconjugation and in developing and manufacturing cADCs, radioimmunoconjugates, and other conjugates. They demonstrated a great deal of flexibility, worked within our budget, and delivered our ADC ahead of schedule.
Partner with a CDMO Built for Bioconjugate Innovation
Whether you’re developing a theranostic radiopharmaceutical, vaccine conjugate, or next-generation bio-conjugates, GBI offers the technical depth, flexibility, and cGMP manufacturing infrastructure to scale your project from concept to clinic.
Contact us today to learn how our bioconjugation services can help bring your conjugated biologic molecule to life.

