Introduction:
In the swiftly evolving landscape of pharmaceuticals, an integrated biologics CDMO plays a pivotal role that cannot be overstated. This holds particularly true for enterprises engaging in the development and production of biologics, a transformative category of drugs that has propelled advancements in the medical field. Biologics, including vaccines, monoclonal antibodies, and cell therapies, have introduced innovative solutions for addressing complex diseases. For companies embarking on the path of biologics’ clinical and commercial manufacturing, the strategic selection of the right biologic CDMO partner stands as a decision that holds the potential to profoundly shape their journey toward success.

The Landscape and Key Considerations of Biologics CDMO Services
Learn more about how to “Reach the patient Quicker with GBI’s Manufacturing Solutions”
The Landscape of Biologic CDMO Services
As the demand for biologics continues to surge, the role of CDMOs providing biologics services has gained prominence. These specialized entities provide a comprehensive spectrum of solutions, spanning from the early phases of development to large-scale manufacturing. In doing so, they alleviate the complexities associated with establishing and managing in-house manufacturing facilities, offering pharmaceutical enterprises a streamlined pathway to bring their biologics to market. With their extensive expertise, utilization of advanced technologies, and in-depth understanding of regulatory compliance, CDMOs facilitate a more efficient and effective route to market readiness.
Key Considerations for Choosing a Biologic CDMO
In the realm of biologics contract manufacturing organizations, several vital factors warrant thoughtful evaluation. These considerations are pivotal in ensuring the seamless transition from clinical trials to full-scale commercial manufacturing, thereby influencing the success of your biologics pharmaceuticals CDMO. This article will provide the essential factors that should guide your decision-making process!

Contract Manufacturing Organization Biologics Experience, Expertise, and Capacity
Expertise and Experience of a Biologic CDMO
At the core of any CDMO’s capabilities lie their expertise and experience in biologics contract manufacturing organizations. Seek a partner with a proven track record of successfully developing and manufacturing an array of biologic drugs. The breadth of their experience across various therapeutic areas underscores their adaptability and proficiency in handling diverse biologics projects. Looking for biologics CDMO companies with platform technologies that match your program will minimize risk through your CMC milestones.
Comprehensive Services of a Biologics Pharmaceutical CDMO
For enterprises engaging with biologics CDMO services, an integrated approach is key. Look for a CDMO that provides a holistic range of services, for example:
Partnering with a CDMO that offers a one-stop solution streamlines operations, simplifies intricacies, and ensures seamless communication across each stage of the manufacturing process.
Regulatory Compliance of Biologics CDMO Companies
Given the stringent regulatory standards governing biologics manufacturing, regulatory compliance is non-negotiable. Prioritize CDMOs with a demonstrated commitment to adhering to international regulatory guidelines. Certifications like cGMP (current Good Manufacturing Practices) serve as indicators of a CDMO’s dedication to upholding the highest standards of quality and safety.
Scalability and Capacity of CDMO Biologics Services
Evaluate both your immediate and long-term manufacturing requirements when assessing a biologics CDMO. An optimal partner should possess the flexibility to accommodate both small-scale clinical production and large-scale commercial manufacturing. This scalability ensures a seamless transition across different developmental phases while maintaining operational efficiency and product quality.

Innovation and Management at CDMO Biologics Services
Technology and Innovation of a Integrated Biologics CDMO
Staying at the forefront of technological advancements is essential for success within the realm of biologics manufacturing. Identify a CDMO that invests in state-of-the-art technologies, as innovations in bioprocessing, analytics, and automation can significantly enhance productivity, yield, and competitiveness within the market.
Project Management and Communication of a Biologics CDMO
CDMO biologics services with effective project management and transparent communication are pivotal for timely delivery and successful collaboration. Throughout the partnership a reputable biologics CDMO should boast the following:
- A dedicated project management team
- Ensure adherence to timelines
- Swift resolution of challenges
- Regular updates
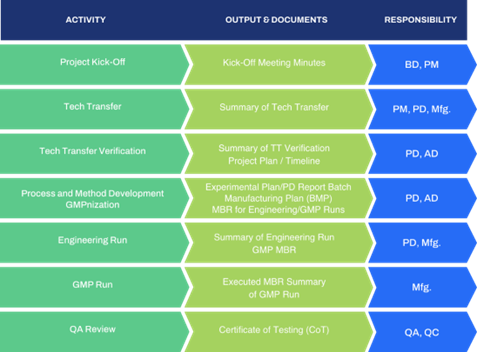
The image above shows GBI’s project management process, click here to learn more.
Quality Control of Biologics Contract Manufacturing Organizations
The quality of biologic products is directly correlated with their safety and efficacy. When considering a CDMO, inquire about their quality control processes, analytical capabilities, and testing methodologies. A robust quality assurance framework ensures consistent product quality throughout the lifecycle.
Conclusions on choosing the right Biologics CDMO
The selection of the right biologics CDMO entails a decision-making process that reverberates through every stage of your product’s journey, spanning from development to market launch. By evaluating potential partners based on their expertise, comprehensive services, regulatory compliance, scalability, technological innovation, project management capabilities, and quality control measures, you can confidently make a well-informed choice.
In the dynamic realm of biologics contract development and manufacturing organizations, collaborating with a CDMO that aligns with your company’s vision and requirements is pivotal. Through this partnership, you can harness advanced capabilities and industry insights, ultimately driving the success of your innovative biologic therapies. As you navigate the intricate landscape of contract manufacturing organization biologics, remember that the right collaboration serves as the cornerstone for bringing transformative biologic products to the patients who stand to benefit the most.
Talk to us about your Biologics Project
Need help with your next biologics manufacturing project? GBI is a one-stop shop that can take your project from Drug substance to Drug Product all under one roof! Contact GBI Biomanufacturing today.

Nick has a track record of developing partnerships with pharmaceutical and biotechnology executives to support the biologic CMC activities required to support their clinical trials and hit development milestones. With a background as a bench scientist specializing in manufacturing scale-ups, Nick brings a unique skillset that bridges the business and technical communications between GBI’s team and external stakeholders. Nick’s recent experience involved business development roles for Tokyo Chemical Industry and Cytovance Biologics. He has a B.S. in chemistry and an MBA from the University of Massachusetts, Lowell.
