

Experience.
Scalability.
Scalable Upstream Processing & Cell Culture Development by a Leading US-based CDMO
As a premier US-based CDMO, GBI specializes in upstream processing and cell culture development for complex biologics. Our expertise spans the development of monoclonal antibodies, recombinant proteins, multispecific antibodies, exosomes, and others —moving seamlessly from R&D to cGMP manufacturing.
When it comes to CDMO companies in the US, GBI is a trusted partner for innovative biopharmaceutical organizations seeking robust, reproducible, and scalable upstream processing solutions aligned with global regulatory standards.
Contact UsComprehensive Cell Culture Development for Biologics
At GBI, we ensure that every biologic molecule project is designed with scalability, cost efficiency, and regulatory compliance in mind. From early-stage cell culture optimization to late-stage development and cGMP manufacturing, our upstream strategies are built to meet stringent quality criteria for identity, purity, potency, safety, and immunogenicity.
As a top CDMO in the US, we support clients through:
- Cell line selection and cell culture optimization
- Animal-free media development
- Growth performance and productivity enhancement
- Process characterization
- Scalability for clinical and commercial manufacturing
Our cGMP manufacturing infrastructure ensures smooth transitions from process development to clinical and commercial production.
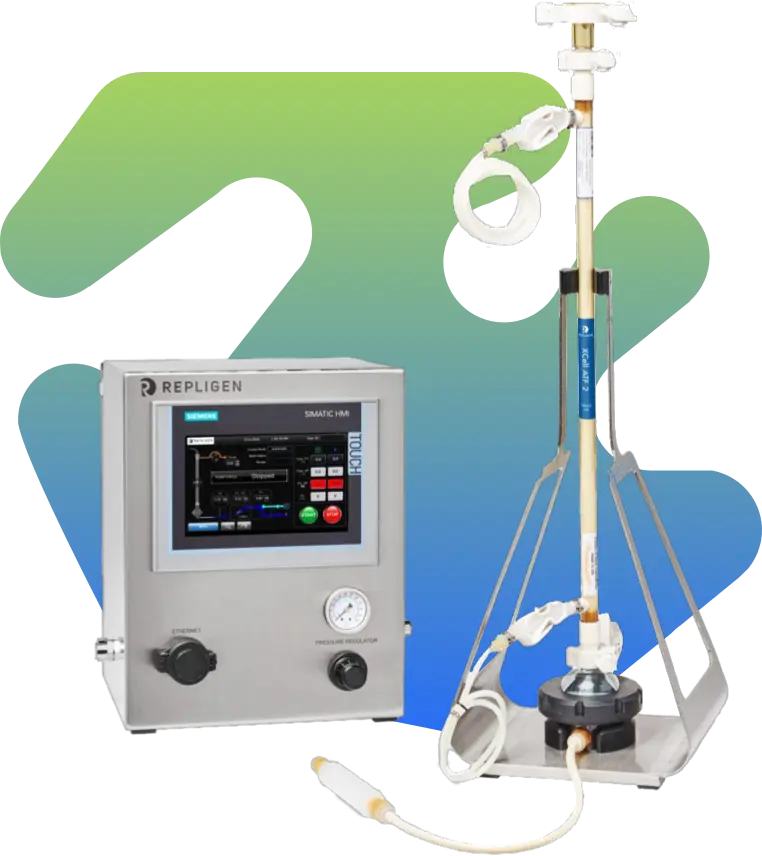
Industry-Leading Upstream Processing Technologies
GBI’s Process Development Lab is equipped with a diverse range of cell culture technologies tailored for flexible scale-up and optimization, including:
- Stationary culture disposable flasks
- Shake flasks, cell factories and spinners
- WAVE bioreactors
- Stirred tank and fed-batch bioreactors
- Perfusion systems
We also support non-bioreactor production projects and provide specialized upstream development services for different bioreactor platforms.

We work with a number of companies around the world to advance our portfolio of drug candidates, and one of our primary collaborators highly recommended GBI based on the track record they had on a number of their projects that GBI had worked on.
Partner with a Trusted US-based CDMO
If you’re looking for one of the most experienced CDMO companies in the US for cell culture-based upstream processing, GBI offers end-to-end support and proven performance.
Contact us today to learn how we can help bring your therapy from cell culture to cGMP production—faster, safer, and smarter.

