Introduction
In the ever-evolving space of biopharmaceuticals, the demand for scalable manufacturing solutions is more pressing than ever. As biotech and biopharma companies work towards bringing innovative therapies to market, they face significant challenges in scaling up production, while maintaining product quality and regulatory compliance. This is where Contract Development and Manufacturing Organizations (CDMOs) specializing in complex biologics play a key role. In this article, we will explore the strategies implemented by biomanufacturing CDMOs to overcome scalability challenges and drive success in the biotech industry.
Uncovering CDMO Strategies for Scalable Biomanufacturing
Understanding Complex Biopharmaceuticals
Biopharmaceuticals are complex medicines made from living cells or organisms. For example, complex biopharmaceuticals include a diverse range of therapeutic modalities, such as monoclonal antibodies, recombinant proteins and cell-based therapies. These innovative therapeutics often require specialized manufacturing processes due to their intricate molecular structures and sensitivity to environmental conditions. The need for personalized medicine of these biologics is at an ultimate rise. Biopharmaceutical CDMOs offer customized solutions tailored to the unique requirements for clients providing therapies to market.
Learn more about “Choosing the Right Monoclonal Antibodies Manufacturer: A Comprehensive Guide” here!
Innovation in Process Development
Process Development is the first pass to a scalable manufacturing process. Biotech CDMOs use Lean Six Sigma methodologies to streamline manufacturing processes and reduce production costs. CDMOs can continuously improve process optimization with Design of Experiments (DoE) to increase product yield, reduce cycle times and enhance product quality. Advanced analytics and modeling tools allow biopharmaceutical CDMOs to identify bottlenecks, optimize resource utilization, and predict outcomes, ensuring efficient and scalable production. By systematic evaluation and eliminating inefficiencies, CDMOs drive operational excellence and ensure consistent delivery of high-quality biopharmaceutical products to the market.
Flexibility & Scalability
Biopharmaceuticals CDMOs enable biotech companies to expedite the transition from preclinical development to clinical manufacturing, by leveraging their expertise in process development and scale-up manufacturing. CDMOs invest in suitable facilities and versatile manufacturing platforms that enable scale-up/down capabilities, allowing adaptation to changing market dynamics and client needs. Rapid reconfiguration and efficient technology transfer processes facilitate seamless integration of manufacturing operations, minimizing time-to-market and maximizing commercial opportunities. Whether scaling up for commercial production or addressing fluctuations in demand, CDMOs provide manufacturing capabilities that accommodate varying batch sizes and production schedules, optimizing resource utilization and operational efficiency. Biotech companies need CDMOs who provide flexible manufacturing services for complex biopharmaceuticals that adapt to evolving market demands and production requirements.
Learn more about how to “Reach the Patient Quicker with GBI’s Manufacturing Solutions” here!
Meeting Regulatory Standards
Compliance with Regulatory Guidelines
Compliance with regulatory requirements is principal in biopharmaceutical manufacturing. Biotech CDMOs must comply with strict regulatory standards in place by global regulatory authorities, including the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). By having comprehensive quality management systems and fulfilling regular audits and inspections, CDMOs ensure compliance with current Good Manufacturing Practices (cGMP) and other regulatory guidelines. Biopharmaceutical CDMOs mitigate regulatory risks and uphold the highest standards of manufacturing success by adhering to stringent guidelines to ensure product safety, efficiency and quality.
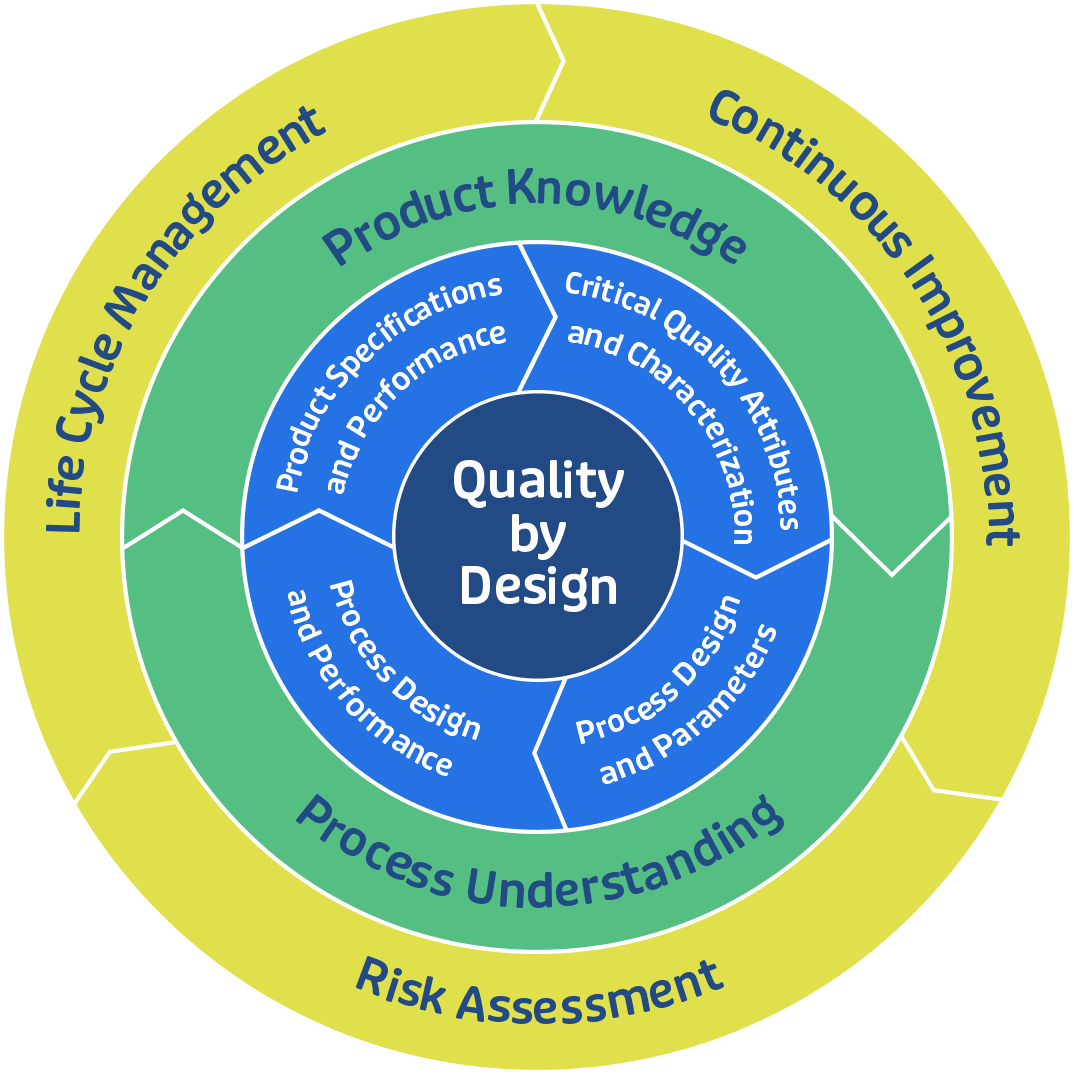
Quality by Design (QbD) Principles
CDMOs embrace Quality by Design (QbD) principles to create robust and scalable manufacturing processes that prioritize product quality and performance attributes. By employing risk-based approaches and process analytical technologies (PAT), CDMOs optimize process parameters and enhance product consistency throughout the manufacturing lifecycle. Biopharmaceutical CDMOs employ rigorous quality control measures to monitor and evaluate product quality throughout the manufacturing process. By maintaining a culture of quality and continuous improvement, CDMOs uphold the highest standards of product quality and safety.
Click here to here from GBI’s Sr. Manager of Quality Control about working in the STEM field!
Validation & Qualifications Protocols by Quality Assurance
Validation and qualification protocols are essential components of biopharmaceutical manufacturing. CDMOs quality assurance personnel perform validation studies and qualification assessments to ensure the reliability and reproducibility of manufacturing processes. Analytical testing, in-process monitoring, and validation protocols are used to assess product attributes, detect deviations, and ensure compliance with specifications. From equipment qualification to analytical method validation, CDMOs adhere to industry best practices to validate critical process parameters and analytical techniques, facilitating regulatory approval and commercialization.
Biopharmaceutical CDMO: Partnering for Success
The Role of a Biopharmaceutical CDMO
Biopharmaceutical CDMOs are strategic partners for biotech and biopharma companies looking to scale up production while mitigating risks and reducing costs. When outsourcing manufacturing to biotech CDMOs, companies can leverage state-of-the-art facilities, advanced technologies, and regulatory expertise without investing in costly infrastructure or hiring specialized personnel. Biotech CDMOs offer manufacturing services for complex biopharmaceuticals, ranging from cell line development and process optimization to large-scale production, sterile fill finish and regulatory compliance. By leveraging expertise in bioprocessing technologies, CDMOs assist biotech companies to navigate the complexities of biopharmaceutical development efficiently.
Advantages of Partnering with a CDMO for Biotech
Biotech CDMOs work closely with clients to understand their specific goals, timelines, and regulatory requirements, providing a coherent and cooperative partnership throughout the development and manufacturing process. With single-use bioreactors to continuous manufacturing systems, these technologies allow CDMOs to scale up biologics manufacturing while maintaining product quality and consistency. They invest in talent development programs and provide ongoing training to qualify employees with the knowledge and skills needed to succeed in a dynamic industry environment. Partnering with a CDMO for biotech also gives Biotech companies access to Subject Matter Experts (SMEs) and overall support in filing an Investigational New Drug (IND) or Biologics License Application (BLA) submission to ensure all requirements are met for a seamless process to market.

Learn more about “Choosing the Right Biologics CDMO for Your Therapeutic Development Needs” here!
Supply Chain Management & Risk Mitigation
Successful partnerships between biotech companies, CDMOs and suppliers hinge on effective communication and collaboration. By establishing strategic partnerships with reliable suppliers and implementing strict quality control measures, CDMOs can minimize supply chain disruption and maintain product integrity throughout the manufacturing process. Proper supply chain management is critical to ensuring uninterrupted production and on-time delivery of biopharmaceutical products. Biomanufacturing CDMOs use robust supply chain strategies to avoid risks associated with raw material sourcing, logistics, and regulatory compliance.
Conclusion
In conclusion, overcoming scalability challenges in biopharmaceutical manufacturing requires a combination of strategic planning, technical expertise, and regulatory compliance. Biomanufacturing CDMOs play a primary role in allowing biotech and biopharma companies to scale up production, accelerate time to market, and deliver innovative therapies to patients worldwide. By partnering with a specialized CDMO, companies can leverage innovative technologies, compliance with regulatory standards, and gain a culture of continuous improvement. CDMOs support biotech companies with navigating the complexities of biopharmaceutical development and achieving commercial excellence. As the demand for complex biopharmaceuticals continues to grow, the role of CDMOs in driving innovation and success in the biopharmaceutical industry will become increasingly indispensable.
Talk to GBI about Your Clinical and Commercial CMC Outsourcing Strategy!
Need a biopharmaceutical CDMO Partner? GBI is a one-stop shop that can take your project from Tech transfer of the gene sequence to Vialed Drug Product (VDP) all under one roof! Contact GBI Biomanufacturing Today!

Yaslin is a dynamic and accomplished professional whose background is in Pharmaceutical Sciences with preclinical expression of drug transporters and its impact on chemical disposition and toxicity. Currently she has been focusing her years of invaluable experience in the biotechnology industry starting with Research and Development work at Assembly Biosciences. Yaslin has showcased her versatility by making significant contributions to the marketing and sales industry, working for Seacret Direct before transitioning to her current role at GBI Biomanufacturing. Fluent in both English and Spanish, she combines linguistic dexterity with a detail-oriented, engaging and determined approach to every task. With a BS in Pharmaceutical Sciences and MBA from the University of Rhode Island, she seamlessly bridges the worlds of science and business together.
